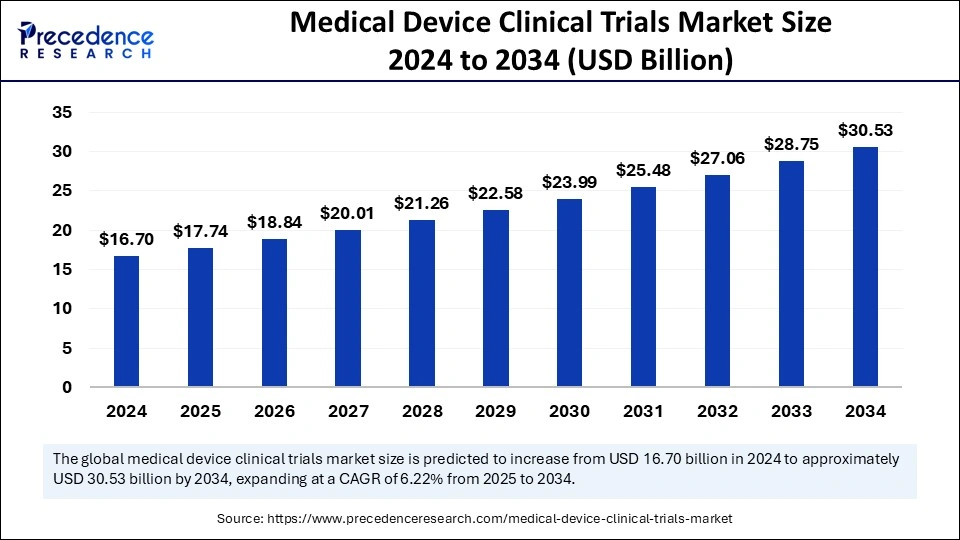
Medical Device Clinical Trials Market Key Takeaways
- North America dominates the global market by generating the largest share of 44% in 2024.
- Asia Pacific is anticipated to emerge as the fastest-growing market during the forecast period of 2025 to 2034.
- By study type, the pivotal study segment dominated the global market in 2024.
- By study type, the feasibility and pilot study segment is anticipated to emerge as the fastest-growing during the forecast period of 2025 to 2034.
- By study design, the interventional segment held the largest market share in 2024.
- By study design, the observational segment is anticipated to grow at the highest CAGR during the forecast period of 2025 to 2034.
- By indication, the cardiovascular devices segment marked its dominance by contributing to the largest share in 2024.
- By indication, the neurology segment is expected to grow at a CAGR of 7.4% during the forecast period of 2025 to 2034.
Market Overview
A medical device clinical trials is a study mainly conducted to evaluate the performance and safety of medical devices before their approval. Various organizations are involved in the process, and they ensure the regulatory standards are fulfilled by the devices.
The medical device clinical trials market is growing rapidly due to the growing popularity of advanced equipment in the healthcare industry, which mainly focuses on patient safety and service outcomes. There are various processes like non-clinical studies, pilot studies, pivotal studies, and post-market surveillance that use various patients for their clinical purposes.
Drivers
Key drivers of the market include the rising burden of chronic diseases, which necessitates the development of novel medical devices to improve patient outcomes. The integration of digital technologies, such as AI, machine learning, and wearable devices, has revolutionized clinical trial processes, enabling real-time data collection, predictive analytics, and personalized treatment approaches. Additionally, the shift towards patient-centric trial designs and the emphasis on regulatory compliance have further propelled market growth.
Opportunities
The market offers substantial opportunities for stakeholders to capitalize on the evolving landscape. The adoption of decentralized clinical trials allows for greater flexibility, reduced costs, and improved patient recruitment, especially in remote or underserved areas.
Emerging economies present untapped potential, with increasing healthcare investments, favorable regulatory reforms, and a growing demand for advanced medical technologies. Strategic partnerships and collaborations can facilitate knowledge sharing, resource optimization, and accelerated device development.
Challenges
Despite the promising prospects, the market faces challenges that need to be addressed. The complexity of regulatory requirements across different regions can lead to delays and increased costs in trial execution. Ensuring data integrity, privacy, and security in the context of digital trials is a critical concern. Additionally, the need for skilled personnel to manage sophisticated technologies and interpret complex data sets poses a challenge, particularly in resource-limited settings.
Regional Insights
North America continues to lead the market, driven by a robust healthcare system, significant R&D investments, and a favorable regulatory environment. Europe maintains a strong presence, with a focus on innovation and quality standards. The Asia-Pacific region is emerging as a key growth area, with countries like China and India investing heavily in healthcare infrastructure and clinical research capabilities. Latin America and the Middle East are also witnessing increased clinical trial activities, supported by government initiatives and international collaborations.
Recent Developments
Recent advancements in the market include the increased adoption of virtual and hybrid clinical trial models, which combine traditional and digital approaches to enhance flexibility and efficiency. Regulatory agencies are updating guidelines to accommodate these new methodologies, ensuring patient safety and data reliability. The use of blockchain technology for secure data management and the application of AI for predictive modeling are gaining traction, offering new avenues for innovation in clinical trials. Additionally, the COVID-19 pandemic has accelerated the adoption of remote monitoring and telemedicine, reshaping the clinical trial landscape.
Medical Device Clinical Trials Market Companies
- Medtron ic
- Siemens Healthineers AG
- Fresenius Medical Care AG
- GE Healthcare
- Koninklijke Philips N.V.
- Danaher Corporation
- Baxter
- Boston Scientific Corporation
- F. Hoffmann La Roche
Segments covered in the report
By Study Type
- Feasibility and Pilot Study
- Pivotal Study
- FDA PMA Application
- Post-Approval Study
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Cardiovascular devices
- Neurology devices
- Orthopedic devices
- Diagnostic imaging
- Anesthesia and Respiratory devices
- Others
By Region
- North America
- Latin America
- Europe
- Asia-pacific
- Middle and East Africa
Ready for more? Dive into the full experience on our website!
