Immunology Biosimilars Market Key Takeaways
- In 2024, North America led the immunology biosimilars market with the highest market share.
- Asia Pacific is projected to register the fastest compound annual growth rate over the forecast period.
- Europe is gradually establishing itself as a significant regional player in the market.
- Inflammatory bowel disease emerged as the leading disease segment in 2024.
- The arthritis segment is anticipated to grow at the fastest pace during the projected period.
- Hospital pharmacies represented the largest share among distribution channels in 2024.
- Retail pharmacies are expected to experience strong growth momentum in the coming years.
Market Overview
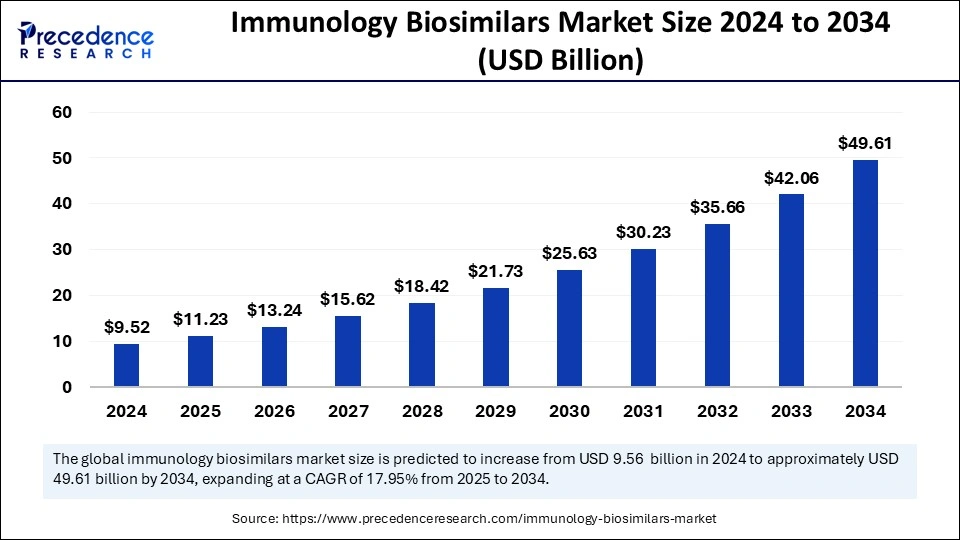
The global immunology biosimilars market is undergoing significant transformation as biologic patent expirations continue to open doors for new entrants. Biosimilars in immunology are designed to be highly similar to reference biologics, offering comparable safety, purity, and efficacy. The immunology biosimilars market is witnessing rapid expansion driven by cost-effectiveness, rising prevalence of autoimmune disorders, and increasing awareness among healthcare professionals. Biologic drugs used to treat conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease have historically been expensive, but biosimilars are changing that narrative by offering competitive alternatives.
Growth in the immunology biosimilars market is being catalyzed by global regulatory frameworks that are becoming increasingly streamlined. Agencies like the FDA and EMA have introduced more structured pathways to approve biosimilars, particularly those targeting immunology indications. These regulations are fostering innovation while ensuring patient safety and efficacy, and the result is a booming market that presents lucrative opportunities for pharmaceutical companies. Market participants are also investing heavily in R&D and clinical trials to meet the required bioequivalence standards, further pushing the growth trajectory of the immunology biosimilars market.
As the demand for affordable treatment options continues to rise globally, especially in emerging markets, biosimilars are becoming the preferred alternative. This shift is propelling the immunology biosimilars market into a new era of accessibility and affordability. With several high-profile biologics nearing patent expiration and multiple biosimilars already on the market, the competitive landscape is intensifying, prompting companies to differentiate themselves not only through price but also through patient support programs and service offerings.
Drivers
One of the primary drivers of the immunology biosimilars market is the increasing incidence of chronic and autoimmune diseases. Conditions such as rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, and ulcerative colitis are seeing rising global prevalence. These diseases require long-term management with biologic therapies, but the high cost of original biologics often poses access issues. Immunology biosimilars offer a cost-effective alternative, thereby increasing treatment penetration and supporting market growth.
The expiration of patents for blockbuster biologic drugs is another significant catalyst in the immunology biosimilars market. Products like Humira (adalimumab) and Enbrel (etanercept) have either lost or are nearing patent protection expiry, creating a window of opportunity for biosimilar manufacturers. This dynamic is encouraging numerous pharmaceutical firms to enter the market with biosimilar versions of these therapies.
Furthermore, government initiatives and reimbursement policies are playing a vital role in promoting biosimilar adoption. In various countries, healthcare systems are under pressure to control rising medical costs, and biosimilars offer an effective way to reduce spending without compromising treatment quality. Favorable reimbursement scenarios and inclusion of biosimilars in national formularies are accelerating their uptake, driving the expansion of the immunology biosimilars market.
Opportunities
The immunology biosimilars market presents immense opportunities in both developed and emerging regions. In developed markets such as North America and Europe, the acceptance of biosimilars is steadily increasing as more clinical data becomes available demonstrating their efficacy and safety. These regions also offer advanced infrastructure and regulatory support, making them ideal for launching new immunology biosimilar products.
Emerging economies represent another major opportunity for the immunology biosimilars market. Countries in Asia-Pacific, Latin America, and the Middle East are seeing increased demand for affordable biologic therapies. With growing healthcare awareness, improvements in infrastructure, and rising disposable incomes, these regions are primed for biosimilar adoption. In particular, nations like India, China, and Brazil are experiencing rapid market penetration, as they seek to bridge the treatment gap in autoimmune conditions.
Digitalization and advancements in biosimilar manufacturing technologies are also creating new growth avenues. Companies are investing in state-of-the-art biologic manufacturing plants that offer higher yields, better scalability, and consistent quality. As biosimilar developers become more adept at creating robust, interchangeable products, the credibility and acceptance of these therapies are likely to increase, further strengthening the position of the immunology biosimilars market.
Challenges
Despite the strong growth outlook, the immunology biosimilars market faces several challenges that could hinder its expansion. One of the foremost challenges is the complexity of biologic drugs. Unlike small-molecule drugs, biologics are made from living cells, and replicating their exact structure is extremely difficult. As a result, biosimilar development requires sophisticated technology, significant investment, and rigorous clinical trials to ensure equivalency, increasing time to market and production costs.
Another barrier in the immunology biosimilars market is physician and patient skepticism. Although biosimilars are designed to be nearly identical to reference biologics, there is still a lack of awareness and confidence among some stakeholders. Education campaigns, real-world evidence, and post-marketing surveillance are essential to build trust and ensure that biosimilars are embraced as viable alternatives.
Legal and intellectual property issues also continue to complicate the landscape. Innovator companies often engage in aggressive patent litigation to delay the entry of biosimilars into the market. Such lawsuits can tie up resources and postpone product launches, affecting the growth of the immunology biosimilars market. Additionally, pricing pressures and the need to negotiate with payers and insurers present challenges in terms of profitability and market penetration.
Regional Insights
North America holds a significant share of the immunology biosimilars market, largely due to the high prevalence of autoimmune diseases and a well-established biologics industry. The United States, in particular, has seen a gradual but steady increase in biosimilar approvals, especially since the introduction of the Biologics Price Competition and Innovation Act (BPCIA). While uptake has been slower compared to Europe, growing support from payers and positive clinical data are changing the narrative.
Europe remains a global leader in the immunology biosimilars market, thanks to its progressive regulatory framework and high adoption rates. The European Medicines Agency (EMA) has been approving biosimilars since 2006, making Europe the most mature biosimilar market globally. Countries like Germany, the UK, and France have robust healthcare systems that actively encourage biosimilar usage, making them key contributors to the market’s expansion.
The Asia-Pacific region is experiencing rapid growth in the immunology biosimilars market. Increasing healthcare expenditure, government initiatives, and a large patient base are all contributing to the market’s rise in countries like India, China, and South Korea. These nations are not only major consumers but are also becoming hubs for biosimilar manufacturing, leveraging low production costs and growing expertise.
In Latin America and the Middle East & Africa, the immunology biosimilars market is gradually gaining traction. Although these regions face challenges like regulatory delays and limited awareness, ongoing reforms and international collaborations are expected to drive growth. Multinational pharmaceutical companies are increasingly looking to these markets for expansion, offering lower-cost alternatives to expensive biologics.
Recent Developments
The immunology biosimilars market has witnessed several noteworthy developments in recent years, ranging from new product approvals to strategic collaborations. One of the most significant events has been the approval and launch of biosimilars for adalimumab and infliximab, two of the most widely used biologics in immunology. These launches have intensified competition and driven down treatment costs in various markets.
Pharmaceutical companies are increasingly entering into partnerships to bolster their position in the immunology biosimilars market. Collaborations between drug manufacturers and contract research organizations (CROs) are enabling faster clinical development and regulatory approval processes. Joint ventures between large pharma and biotech firms are also becoming common as companies look to share R&D costs and accelerate time-to-market.
Another trend reshaping the immunology biosimilars market is the rise of interchangeability designations. As more biosimilars achieve interchangeable status, they can be substituted for reference biologics at the pharmacy level without prescriber intervention. This development is expected to significantly boost adoption rates and enhance market competitiveness.
Immunology Biosimilars Market Companies
- Novartis AG
- Pfizer Inc.
- Mylan N.V.
- AbbVie Inc.
- STADA Arzneimittel AG
- Celltrion, Inc.
- KBI Biopharma, Inc.
- Amgen, Inc
Segments Covered in the Report
By Disease
- Inflammatory Bowel Disease
- Arthritis
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!
