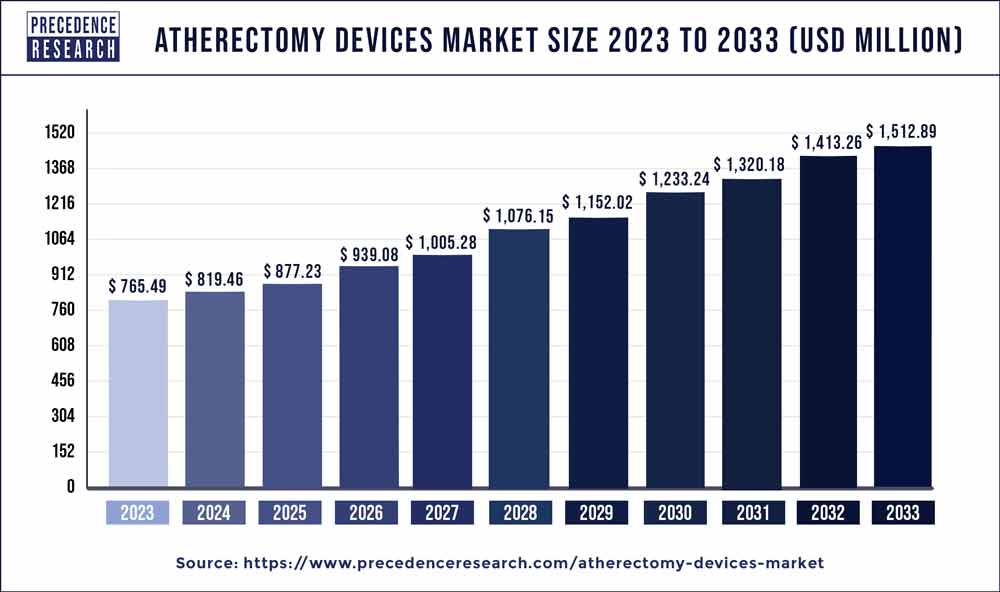
The global atherectomy devices market size was valued at USD 765.49 million in 2023 and is estimated to reach around USD 1,512.89 million by 2033, growing at a CAGR of 7.05% from 2024 to 2033.
Key Points
- North America led the market with the biggest market share of 45% in 2023.
- Asia Pacific is projected to expand at the fastest rate during the forecast period of 2024-2033.
- By device type, the drug-coated balloons (DCBs) segment held the largest share of the market in 2023.
- By device type, the intravascular ultrasound (IVUS) catheter segment is expected to show the fastest growth.
- By application type, the peripheral artery disease segment held the dominating share of the market in 2023.
- By application type, the coronary artery disease segment represents another highly influential segment for the forecast period.
- By end-user, the cardiac catheterization labs segment held the dominating share of the market in 2023.
- By end-user, the interventional radiology departments segment is expected to witness a significant rate of expansion during the forecast period.
Report Summary
The global atherectomy devices market report provides a Point-by-Point and In-Depth analysis of global market size, regional and country-level market size, market share, segmentation market growth, competitive landscape, sales analysis, opportunities analysis, strategic market growth analysis, the impact of domestic and global market key players, value chain optimization, trade regulations, recent developments, product launches, area marketplace expanding, and technological innovations.
The study offers a comprehensive analysis on diverse features, including production capacities, demand, product developments, revenue generation, and sales in the atherectomy devices market across the globe.
A comprehensive estimate on the atherectomy devices market has been provided through an optimistic scenario as well as a conservative scenario, taking into account the sales of atherectomy devices during the forecast period. Price point comparison by region with global average price is also considered in the study.
Download Access to a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/3901
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 7.05% |
| Global Market Size in 2023 | USD 765.49 Million |
| Global Market Size by 2033 | USD 1,512.89 Million |
| U.S. Market Size in 2023 | USD 241.13 Million |
| U.S. Market Size by 2033 | USD 476.56 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Devices Type, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Key Highlights:
Reports Coverage: It incorporates key market sections, key makers secured, the extent of items offered in the years considered, worldwide containerized atherectomy devices market and study goals. Moreover, it contacts the division study gave in the report based on the sort of item and applications.
Market Outline: This area stresses the key investigations, market development rate, serious scene, market drivers, patterns, and issues notwithstanding the naturally visible pointers.
Market Production by Region: The report conveys information identified with import and fare, income, creation, and key players of every single local market contemplated are canvassed right now.
Also Read: Green Cement Market Size to Hold USD 1,046.76 Million by 2033
Market Players
The report includes the profiles of key atherectomy devices market companies along with their SWOT analysis and market strategies. In addition, the report focuses on leading industry players with information such as company profiles, components and services offered, financial information, key development in past five years.
Recent Developments
- In October 2023, Cardio Flow, Inc., announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its FreedomFlow® Orbital Atherectomy Peripheral Platform.
- In September 2023, Avinger Inc., a commercial-stage medical device company, announced the full commercial launch of its Tigereye® ST next-generation image-guided chronic total occlusion (CTO) crossing system.
Atherectomy Devices Market Companies
- Abbott Laboratories
- Boston Scientific Corporation
- BD
- Cardinal Health Inc.
- Koninklijke Philips NV
- Medtronic Plc
- Terumo Corporation
- Avinger
- Cardiovascular Systems
- Ra Medical Systems
Segments Covered in the Report
By Devices Type
- Atherectomy Devices
- Angioplasty Balloon Catheters
- Stents (Bare-Metal, Drug-Eluting, Bioresorbable)
- Intravascular Ultrasound (IVUS) Catheters
- Optical Coherence Tomography (OCT) Catheters
- Drug-Coated Balloons
- Embolic Protection Devices
- Thrombectomy Devices
- Aortic Stent Grafts
- Endovascular Grafts
- Laser Atherectomy Devices
- Orbital Atherectomy Systems
- Rotational Atherectomy Devices
- Directional Atherectomy Devices
- Chronic Total Occlusion (CTO) Devices
By Application
- Peripheral Artery Disease (PAD) Treatment Devices
- Coronary Artery Disease (CAD) Intervention Devices
- Carotid Artery Disease Intervention Devices
- Renal Artery Disease Intervention Devices
- Aortic Atherosclerosis Intervention Devices
By End-user
- Cardiac Catheterization Labs
- Interventional Radiology Departments
- Vascular Surgery Centers
- Cardiology Clinics
- Academic Research Institutions
Regional Segmentation
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Southeast Asia and Rest of APAC)
- Latin America (Brazil and Rest of Latin America)
- Middle East and Africa (GCC, North Africa, South Africa, Rest of MEA)
Research Methodology
Secondary Research
It involves company databases such as Hoover’s: This assists us to recognize financial information, the structure of the market participants and industry’s competitive landscape.
The secondary research sources referred in the process are as follows:
- Governmental bodies, and organizations creating economic policies
- National and international social welfare institutions
- Company websites, financial reports and SEC filings, broker and investor reports
- Related patent and regulatory databases
- Statistical databases and market reports
- Corporate Presentations, news, press release, and specification sheet of Manufacturers
Primary Research
Primary research includes face-to-face interviews, online surveys, and telephonic interviews.
- Means of primary research: Email interactions, telephonic discussions and Questionnaire-based research etc.
- In order to validate our research findings and analysis, we conduct primary interviews of key industry participants. Insights from primary respondents help in validating the secondary research findings. It also develops Research Team’s expertise and market understanding.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Atherectomy Devices Market
5.1. COVID-19 Landscape: Atherectomy Devices Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Atherectomy Devices Market, By Devices Type
8.1. Atherectomy Devices Market Revenue and Volume, by Devices Type, 2024-2033
8.1.1 Atherectomy Devices
8.1.1.1. Market Revenue and Volume Forecast (2021-2033)
8.1.2. Angioplasty Balloon Catheters
8.1.2.1. Market Revenue and Volume Forecast (2021-2033)
8.1.3. Stents (Bare-Metal, Drug-Eluting, Bioresorbable)
8.1.3.1. Market Revenue and Volume Forecast (2021-2033)
8.1.4. Intravascular Ultrasound (IVUS) Catheters
8.1.4.1. Market Revenue and Volume Forecast (2021-2033)
8.1.5. Optical Coherence Tomography (OCT) Catheters
8.1.5.1. Market Revenue and Volume Forecast (2021-2033)
8.1.6. Drug-Coated Balloons
8.1.6.1. Market Revenue and Volume Forecast (2021-2033)
8.1.7. Embolic Protection Devices
8.1.7.1. Market Revenue and Volume Forecast (2021-2033)
8.1.8. Thrombectomy Devices
8.1.8.1. Market Revenue and Volume Forecast (2021-2033)
8.1.9. Aortic Stent Grafts
8.1.9.1. Market Revenue and Volume Forecast (2021-2033)
8.1.10. Endovascular Grafts
8.1.10.1. Market Revenue and Volume Forecast (2021-2033)
8.1.11. Laser Atherectomy Devices
8.1.11.1. Market Revenue and Volume Forecast (2021-2033)
8.1.12. Orbital Atherectomy Systems
8.1.12.1. Market Revenue and Volume Forecast (2021-2033)
8.1.13. Rotational Atherectomy Devices
8.1.13.1. Market Revenue and Volume Forecast (2021-2033)
8.1.14. Directional Atherectomy Devices
8.1.14.1. Market Revenue and Volume Forecast (2021-2033)
8.1.15. Chronic Total Occlusion (CTO) Devices
8.1.15.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 9. Global Atherectomy Devices Market, By Application
9.1. Atherectomy Devices Market Revenue and Volume, by Application, 2024-2033
9.1.1. Peripheral Artery Disease (PAD) Treatment Devices
9.1.1.1. Market Revenue and Volume Forecast (2021-2033)
9.1.2. Coronary Artery Disease (CAD) Intervention Devices
9.1.2.1. Market Revenue and Volume Forecast (2021-2033)
9.1.3. Carotid Artery Disease Intervention Devices
9.1.3.1. Market Revenue and Volume Forecast (2021-2033)
9.1.4. Renal Artery Disease Intervention Devices
9.1.4.1. Market Revenue and Volume Forecast (2021-2033)
9.1.5. Aortic Atherosclerosis Intervention Devices
9.1.5.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 10. Global Atherectomy Devices Market, By End-user
10.1. Atherectomy Devices Market Revenue and Volume, by End-user, 2024-2033
10.1.1. Cardiac Catheterization Labs
10.1.1.1. Market Revenue and Volume Forecast (2021-2033)
10.1.2. Interventional Radiology Departments
10.1.2.1. Market Revenue and Volume Forecast (2021-2033)
10.1.3. Vascular Surgery Centers
10.1.3.1. Market Revenue and Volume Forecast (2021-2033)
10.1.4. Cardiology Clinics
10.1.4.1. Market Revenue and Volume Forecast (2021-2033)
10.1.5. Academic Research Institutions
10.1.5.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 11. Global Atherectomy Devices Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.1.4. U.S.
11.1.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2. Europe
11.2.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.4. UK
11.2.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.5. Germany
11.2.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.6. France
11.2.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3. APAC
11.3.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.4. India
11.3.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.5. China
11.3.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.6. Japan
11.3.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4. MEA
11.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.4. GCC
11.4.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.5. North Africa
11.4.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.6. South Africa
11.4.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5. Latin America
11.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5.4. Brazil
11.5.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
Chapter 12. Company Profiles
12.1. Abbott Laboratories
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Boston Scientific Corporation
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. BD
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Cardinal Health Inc.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Koninklijke Philips NV
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Medtronic Plc
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Terumo Corporation
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Avinger
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Cardiovascular Systems
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Ra Medical Systems
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com
