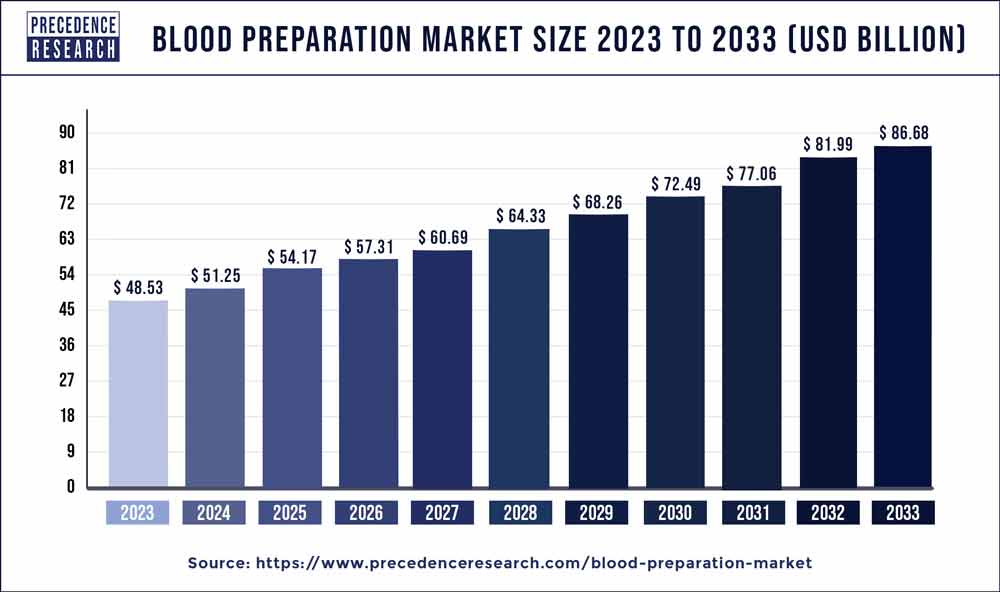
The global blood preparation market size reached USD 48.53 billion in 2023 and is expected to grow around USD 81.99 billion by 2032, at a CAGR of 6% during the forecast period from 2023 to 2032.
Key Takeaways
- North America contributed more than 45% of revenue share in 2022.
- Asia Pacific is expected to witness the fastest growth rate in the blood preparation market during the forecast period.
- By product, the whole blood segment held the largest share of the blood preparation market in 2022.
- The blood derivatives segment is expected to grow at a significant compounded annual growth (CAGR) rate during the forecast period.
- By application, the thrombocytosis segment accounted for a larger share in 2022.
- The angina blood vessel complications segment is estimated to grow with a notable CAGR during the study period till 2032.
The Blood Preparation Market encompasses a diverse range of products and services aimed at ensuring the safety and efficacy of blood transfusions and related medical procedures. This sector plays a crucial role in healthcare by providing blood components, such as red blood cells, plasma, and platelets, prepared to meet specific medical requirements. The market has witnessed steady growth in recent years, driven by factors such as increasing demand for blood and blood components, advancements in blood processing technologies, and a rising emphasis on ensuring the quality of blood products.
Get a Sample: https://www.precedenceresearch.com/sample/3551
Several factors contribute to the growth of the Blood Preparation Market. Technological advancements have led to more efficient and precise methods of blood separation, storage, and testing, enhancing the overall safety of blood products. Additionally, the growing prevalence of chronic diseases and surgical procedures has increased the demand for blood transfusions, driving the need for reliable and standardized blood preparation methods. The expansion of healthcare infrastructure, especially in developing regions, further contributes to market growth by increasing access to blood preparation services.
Furthermore, the market is influenced by regulatory initiatives aimed at enhancing the safety and quality of blood products. Stringent regulations and guidelines set by health authorities push companies in the Blood Preparation Market to adopt advanced technologies and adhere to rigorous quality standards. This not only ensures patient safety but also fosters trust among healthcare professionals and patients, driving the market forward.
Blood Preparation Market Scope
| Report Coverage | Details |
| Growth Rate from 2023 to 2032 | CAGR of 6% |
| Market Size in 2023 | USD 48.53 Billion |
| Market Size by 2032 | USD 81.99 Billion |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Product, By Antithrombotic and Anticoagulants Type, By Application, and By End User |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Read More: Molecular Biology Enzymes Market Size, Growth, Report 2032
- By Product: The Blood Preparation Market is characterized by a range of products designed to meet different medical needs. This includes blood components such as red blood cells, plasma, platelets, and whole blood. Additionally, various blood derivatives and products like immunoglobulins, albumin, and clotting factor concentrates play a crucial role in addressing specific medical conditions.
- By Antithrombotic and Anticoagulants Type: Within the Blood Preparation Market, antithrombotic and anticoagulant agents play a pivotal role in preventing and managing blood clotting disorders. These substances are classified into different types, such as antiplatelet agents, anticoagulant drugs, and thrombolytics. Each type serves distinct therapeutic purposes, ranging from preventing arterial and venous thrombosis to dissolving existing blood clots.
- By Application: The applications of blood preparations span a broad spectrum of medical scenarios. They are extensively used in the treatment of various diseases and conditions, including cardiovascular diseases, hematological disorders, and autoimmune diseases. Furthermore, blood preparations find application in surgical procedures, trauma care, and emergency medicine, highlighting their versatility and significance in diverse healthcare settings.
- By End User: The end user segment of the Blood Preparation Market encompasses a wide array of healthcare institutions and facilities. Hospitals and clinics are primary consumers, utilizing blood preparations in routine medical procedures and emergency situations. Additionally, blood banks and diagnostic laboratories are integral end users, ensuring a stable supply of blood components for therapeutic and diagnostic purposes. The pharmaceutical industry is also a key player, involved in the development and production of blood preparation products.
Reasons to Purchase this Report:
- Comprehensive market segmentation analysis incorporating qualitative and quantitative research, considering the impact of economic and policy factors.
- In-depth regional and country-level analysis, examining the demand and supply dynamics that influence market growth.
- Market size in USD million and volume in million units provided for each segment and sub-segment.
- Detailed competitive landscape, including market share of major players, recent projects, and strategies implemented over the past five years.
- Comprehensive company profiles encompassing product offerings, key financial information, recent developments, SWOT analysis, and employed strategies by major market players.
Recent Developments
- In May 2023, with $50 million from the New York Blood Center, NYBC Ventures was unveiled as one of the first venture funds focusing exclusively on advancing new blood and cell-based therapies. Thus, increasing funds for new blood and cell-based therapies is expected to contribute to the promising growth of the blood preparation market.
- In February 2023, Terumo Blood and Cell Technologies announced the FDA clearance and official launch of its IMUGARD® WB Platelet Pooling Set. This new platelet pooling set supports extended shelf life of whole blood-derived platelets from 5 to 7 days. IMUGARD is the first platelet pooling set approved for 7-day storage in the United States.
- In November 2022, the RESTORE clinical trial (REcovery and survival of STem cell Originated REd cells) was initiated by the NIHR Blood and Transplant Research Unit. Researchers stated that if this clinical trial of lab-grown red blood cells is proven effective and safe, it can revolutionize treatments for people with rare blood types.
Blood Preparation Market Players
- AstraZeneca plc
- Baxter International Inc
- Bristol-Myers Squibb Company
- Celgene Corp.
- GlaxoSmithKline PLC
- Leo Pharma A/S
- Pfizer, Inc.
- Portola Pharmaceuticals, Inc.
- Sanofi
- Shandong East Chemical Industry Co.
- Xiamen Hisunny Chemical Co., LTD
Segments Covered in the Report
By Product
- Whole blood
- Granulocytes
- Red blood cells
- Plasma
- Platelets
- Blood components
- Leukocyte reduced red blood cells
- Packed red blood cells
- Frozen plasma
- Platelet concentrates
- Cryoprecipitate
- Blood derivatives
By Antithrombotic and Anticoagulants Type
- Platelet aggregation inhibitors
- Glycoprotein inhibitors
- ADP antagonists
- COX inhibitors
- Others
- Fibrinolytics
- Streptokinase
- Tissue plasminogen activator (tPA)
- Urokinase
- Anticoagulants
- Heparins
- Low molecular weight heparin (LMWH)
- Unfractionated heparin
- Ultra-low molecular weight heparin
- Direct thrombin inhibitors
- Vitamin K antagonists
- Direct factor Xa inhibitors
- Heparins
By Application
- Thrombocytosis
- Renal impairment
- Pulmonary embolism
- Angina blood vessel complications
- Others
By End User
- Clinics
- Hospitals
- Diagnostic centers
- Research labs
- Blood banks
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Blood Preparation Market
5.1. COVID-19 Landscape: Blood Preparation Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Blood Preparation Market, By Product
8.1. Blood Preparation Market Revenue and Volume Forecast, by Product, 2023-2032
8.1.1. Whole blood
8.1.1.1. Market Revenue and Volume Forecast (2020-2032)
8.1.2. Blood components
8.1.2.1. Market Revenue and Volume Forecast (2020-2032)
8.1.3. Blood derivatives
8.1.3.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 9. Global Blood Preparation Market, By Antithrombotic and Anticoagulants Type
9.1. Blood Preparation Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type, 2023-2032
9.1.1. Platelet aggregation inhibitors
9.1.1.1. Market Revenue and Volume Forecast (2020-2032)
9.1.2. Fibrinolytics
9.1.2.1. Market Revenue and Volume Forecast (2020-2032)
9.1.3. Anticoagulants
9.1.3.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 10. Global Blood Preparation Market, By Application
10.1. Blood Preparation Market Revenue and Volume Forecast, by Application, 2023-2032
10.1.1. Thrombocytosis
10.1.1.1. Market Revenue and Volume Forecast (2020-2032)
10.1.2. Renal impairment
10.1.2.1. Market Revenue and Volume Forecast (2020-2032)
10.1.3. Pulmonary embolism
10.1.3.1. Market Revenue and Volume Forecast (2020-2032)
10.1.4. Angina blood vessel complications
10.1.4.1. Market Revenue and Volume Forecast (2020-2032)
10.1.5. Others
10.1.5.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 11. Global Blood Preparation Market, By End User
11.1. Blood Preparation Market Revenue and Volume Forecast, by End User, 2023-2032
11.1.1. Clinics
11.1.1.1. Market Revenue and Volume Forecast (2020-2032)
11.1.2. Hospitals
11.1.2.1. Market Revenue and Volume Forecast (2020-2032)
11.1.3. Diagnostic centers
11.1.3.1. Market Revenue and Volume Forecast (2020-2032)
11.1.4. Research labs
11.1.4.1. Market Revenue and Volume Forecast (2020-2032)
11.1.5. Blood banks
11.1.5.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 12. Global Blood Preparation Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.1.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.1.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.1.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.1.5. U.S.
12.1.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.1.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.1.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.1.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.1.6.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.1.6.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.1.6.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.2. Europe
12.2.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.2.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.2.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.2.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.2.5. UK
12.2.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.2.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.2.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.2.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.2.6. Germany
12.2.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.2.6.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.2.6.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.2.6.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.2.7. France
12.2.7.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.2.7.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.2.7.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.2.7.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.2.8.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.2.8.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.2.8.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.3. APAC
12.3.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.3.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.3.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.3.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.3.5. India
12.3.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.3.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.3.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.3.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.3.6. China
12.3.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.3.6.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.3.6.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.3.6.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.3.7. Japan
12.3.7.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.3.7.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.3.7.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.3.7.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.3.8.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.3.8.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.3.8.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.4. MEA
12.4.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.4.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.4.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.4.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.4.5. GCC
12.4.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.4.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.4.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.4.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.4.6. North Africa
12.4.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.4.6.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.4.6.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.4.6.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.4.7. South Africa
12.4.7.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.4.7.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.4.7.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.4.7.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.4.8.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.4.8.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.4.8.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.5. Latin America
12.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.5.5. Brazil
12.5.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.5.5.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.5.5.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.5.5.4. Market Revenue and Volume Forecast, by End User (2020-2032)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
12.5.6.2. Market Revenue and Volume Forecast, by Antithrombotic and Anticoagulants Type (2020-2032)
12.5.6.3. Market Revenue and Volume Forecast, by Application (2020-2032)
12.5.6.4. Market Revenue and Volume Forecast, by End User (2020-2032)
Chapter 13. Company Profiles
13.1. AstraZeneca plc
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Baxter International Inc
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Bristol-Myers Squibb Company
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Celgene Corp.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. GlaxoSmithKline PLC
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Leo Pharma A/S
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Pfizer, Inc.
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Portola Pharmaceuticals, Inc.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Sanofi
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Shandong East Chemical Industry Co.
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
