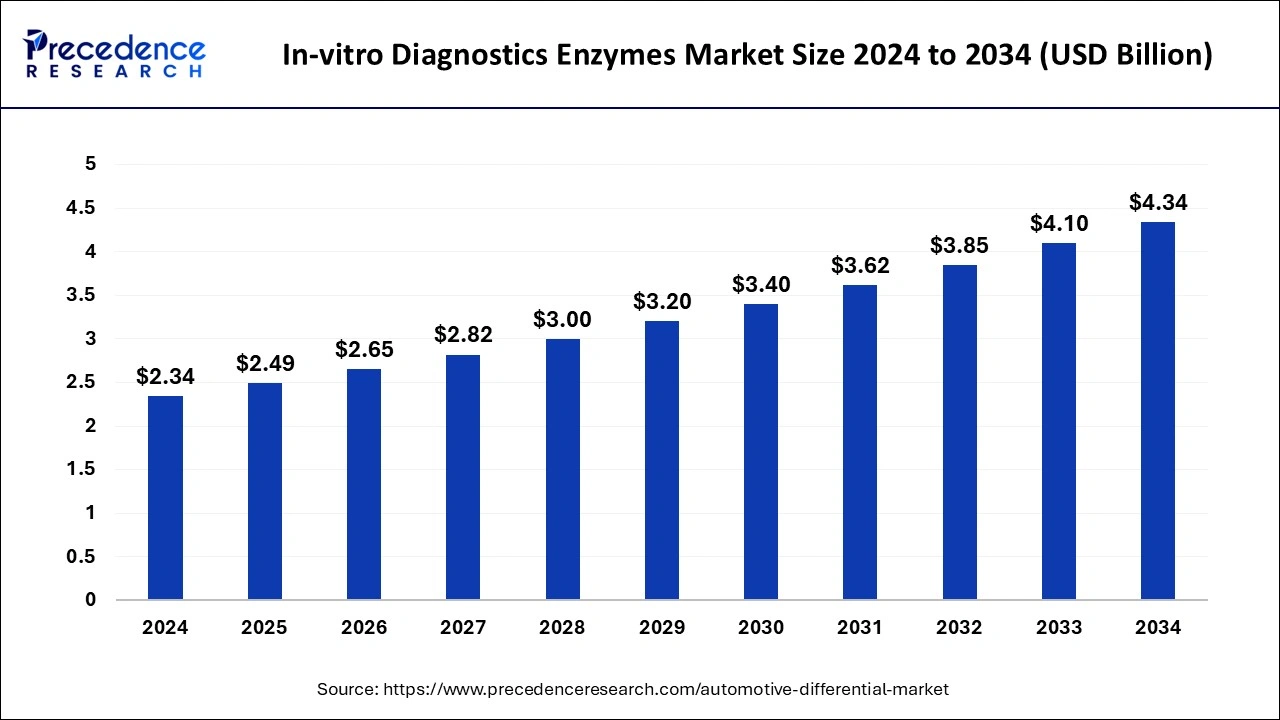
The global in-vitro diagnostics enzymes market size accounted for USD 2.20 billion in 2023 and is expected to attain around USD 4.10 billion by 2033, growing at a CAGR of 6.42% from 2024 to 2033.
Key Points
- The North America in-vitro diagnostics enzymes market size was estimated at USD 920 million in 2023 and is projected to surpass around USD 1,720 million by 2033.
- North America dominated the in-vitro diagnostics enzymes market in 2023 with a revenue share of 42%.
- Asia Pacific is expected to grow at the fastest rate during the forecast period.
- By enzyme type, the polymerase and transcriptase segment has captured a market share of 37% in 2023.
- By end-user, the hospitals and diagnostic segment has held a market share of over 41.8% in 2023.
- By technology, the histology assays segment has accounted market share of 44% in 2023.
- By disease, the infectious disease segment dominated the market in 2023.
The in-vitro diagnostics (IVD) enzymes market encompasses enzymes used in the diagnosis of diseases and conditions outside the living body. These enzymes are essential for various diagnostic tests such as blood glucose monitoring, pregnancy tests, and infectious disease assays. The market is experiencing growth due to advancements in biotechnology and an increasing prevalence of chronic diseases.
Get a Sample: https://www.precedenceresearch.com/sample/4095
Growth Factors
The growth of the IVD enzymes market is primarily driven by the rising demand for accurate and efficient diagnostic tests, especially for chronic conditions like diabetes, cardiovascular diseases, and cancer. Technological advancements in diagnostic methods and the development of novel enzymes for specific tests also contribute to market expansion. Furthermore, the aging population and an increased focus on preventive healthcare are fueling the market’s growth.
Regional Insights
North America leads the in-vitro diagnostics enzymes market due to its well-established healthcare infrastructure, high healthcare expenditure, and a strong focus on research and development. Europe follows, driven by increasing awareness and adoption of advanced diagnostic tests. Asia-Pacific is anticipated to witness significant growth, fueled by a rising population, improved healthcare access, and increasing investments in healthcare infrastructure.
In-vitro Diagnostics Enzymes Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 6.42% |
| Global Market Size in 2023 | USD 2.20 Billion |
| Global Market Size in 2024 | USD 2.34 Billion |
| Global Market Size by 2033 | USD 4.10 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Enzyme Type, By Disease Type, By Technology Type, and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
In-vitro Diagnostics Enzymes Market Dynamics
Drivers
Key drivers of the IVD enzymes market include the growing prevalence of chronic diseases, advancements in diagnostic technologies, and increased healthcare spending. Additionally, the adoption of point-of-care diagnostics and the need for personalized medicine are encouraging the use of IVD enzymes. Rising government initiatives to improve healthcare facilities and diagnostics capabilities also contribute to market growth.
Opportunities
There are opportunities for growth in the IVD enzymes market, particularly in emerging markets where healthcare infrastructure is improving. The development of novel diagnostic tests using enzymes and the increasing focus on molecular diagnostics provide avenues for market expansion. Collaborations between diagnostic companies and research institutions may lead to innovative products and new applications for IVD enzymes.
Challenges
Despite the market’s growth, there are challenges such as regulatory hurdles and the high cost of developing new diagnostic tests. Maintaining enzyme stability and activity for various diagnostic applications can also be complex. Additionally, market players face competition from alternative diagnostic methods and technologies. Intellectual property issues and pricing pressures may also pose challenges for companies in the IVD enzymes market.
Read Also: Electronic Adhesives Market Size to Attain USD 12.40 Bn by 2033
Recent Developments
- In June 2023, GenWorks Health, a Bengaluru-based startup, launched an IVD test for malaria and dengue. This test includes rapid card test kits.
- In September 2023, NeoDX Biotech lab launched an IVD kit for detecting autoimmune disorders. It’s a real-time PCR- a technology-based kit to improve healthcare service and enhance testing abilities.
In-vitro Diagnostics Enzymes Market Companies
- Biocatalysts Ltd.
- Amicogen
- Dyadic International
- BBI Solutions
- Affymetrix
- American Laboratories
- Merck KGaA
- Codexis, Inc.
- F. Hoffmann-La Roche Ltd.
- Amano Enzyme Inc.
- Advanced Enzymes Technologies Ltd.
Segments Covered in the Report
By Enzyme Type
- Proteases
- Polymerase & Transcriptase
- Ribonuclease
- Others
BY Disease Type
- Infectious Disease
- COVID-19 Testing
- Hepatitis
- HIV
- Others
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune diseases
- Others
By Technology Type
- Histology Assays
- Molecular Diagnostics
- PCR Assays
- NGS Assays
- Others
- Clinical Chemistry
By End-use
- Pharma & Biotech
- Hospital & Diagnostic Labs
- Contract Research Organizations (CROs)
- Academic Labs
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
