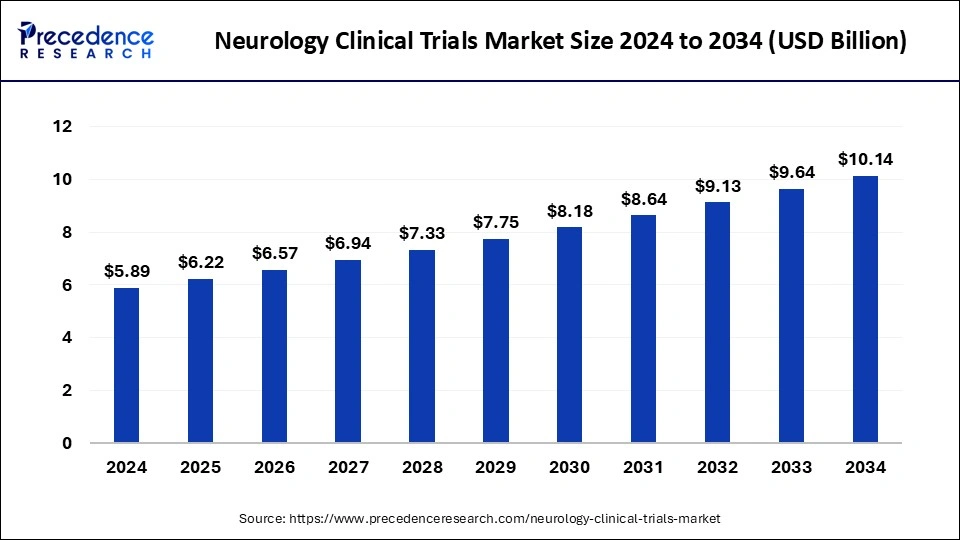
The global neurology clinical trials market size was exhibited at USD 5.58 billion in 2023 and is predicted to rake around USD 9.64 billion by 2033, expanding at a CAGR of 5.62% from 2024 to 2033.
Neurology Clinical Trials Market Key Points
- North America dominated the neurology clinical trials market with the largest revenue share of 48% in 2023.
- Asia Pacific is estimated to grow at a solid CAGR of 5.93% during the forecast period of 2024-2033.
- By phase, the phase II segment has generated more than 47% of revenue share in 2023.
- By phase, the phase III segment is projected to expand at a CAGR of 5.62% during the forecast period.
- By indication, the epilepsy segment has recorded more than 23% of revenue share in 2023.
- By indication, the huntington’s disease segment growing at a CAGR of 6.04% during the forecast period.
- By study design, the interventional segment dominated the market with the biggest revenue share of 96% in 2023.
- By study design, the observational segment is expected to grow at a CAGR of 5.83% during the forecast period.
The Neurology Clinical Trials Market focuses on the evaluation of new treatments, drugs, and therapeutic approaches to manage neurological disorders such as Alzheimer’s disease, Parkinson’s disease, epilepsy, multiple sclerosis, and other neurodegenerative conditions. These trials are critical in advancing medical knowledge and developing effective treatments for a wide range of neurological diseases, which affect millions globally. The market is driven by increasing prevalence of these conditions, advancements in neuroimaging techniques, and a growing emphasis on personalized medicine. With the aging population and the rise in neurodegenerative diseases, there is a significant push towards innovation and improved therapeutic interventions.
Get a Sample: https://www.precedenceresearch.com/sample/4657
Region Insights
The global Neurology Clinical Trials Market is segmented into several key regions: North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America, particularly the United States, dominates the market due to its advanced healthcare infrastructure, significant investments in research and development, and presence of major pharmaceutical companies. Europe follows closely, with substantial contributions from countries like Germany, the UK, and France, where robust clinical research frameworks exist. The Asia Pacific region is witnessing rapid growth, driven by increasing healthcare expenditures, rising patient populations, and the expansion of clinical trial networks in countries like China, India, and Japan. Latin America and the Middle East & Africa, while smaller in market size, are gradually becoming important regions for neurology clinical trials due to improving healthcare systems and growing awareness of neurological diseases.
Trends
Several trends are shaping the Neurology Clinical Trials Market. There is a growing emphasis on personalized medicine, where treatments are tailored to individual genetic profiles, improving the efficacy and safety of therapies. The use of artificial intelligence (AI) and machine learning (ML) in clinical trials is increasing, aiding in patient recruitment, data analysis, and prediction of trial outcomes. Additionally, virtual and decentralized clinical trials have gained traction, especially post the COVID-19 pandemic, allowing for remote patient monitoring and data collection. This trend is enhancing patient participation and reducing the logistical burdens of traditional clinical trials.
Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 9.64 Billion |
| Market Size in 2023 | USD 5.58 Billion |
| Market Size in 2024 | USD 5.89 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 5.62% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Indication, Study Design, Phase, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Neurology Clinical Trials Market Dynamics
Drivers
The primary drivers of the Neurology Clinical Trials Market include the increasing prevalence of neurological disorders and the aging global population, which is more susceptible to neurodegenerative diseases. Technological advancements in neuroimaging and diagnostic tools are facilitating better patient selection and monitoring during trials. Significant investments by pharmaceutical companies and government bodies in research and development are also propelling market growth. Moreover, regulatory agencies are increasingly providing frameworks and incentives for conducting clinical trials, thereby fostering an environment conducive to innovation in neurology therapeutics.
Opportunities
There are numerous opportunities within the Neurology Clinical Trials Market. The advent of biomarkers and companion diagnostics offers potential for more precise and targeted therapies, enhancing trial outcomes and treatment effectiveness. Emerging markets in Asia Pacific, Latin America, and the Middle East present significant growth opportunities due to increasing healthcare investments and patient populations. Collaborations between pharmaceutical companies, research institutions, and healthcare providers are also expected to drive innovation and accelerate the development of new treatments. Additionally, advancements in digital health technologies and telemedicine are opening new avenues for conducting and managing clinical trials more efficiently.
Challenges
Despite the promising outlook, the Neurology Clinical Trials Market faces several challenges. High costs associated with conducting clinical trials, particularly in developed regions, can be a significant barrier. Recruitment and retention of participants, especially in long-term trials for chronic neurological conditions, remain challenging. Regulatory complexities and varying requirements across different regions can also pose hurdles for global trials. Furthermore, the complexity of neurological disorders, with their multifactorial etiologies and varying manifestations, complicates the design and execution of effective clinical trials. Addressing these challenges requires coordinated efforts from stakeholders across the healthcare ecosystem.
Read Also: Menstrual Health Apps Market Size to Rake USD 9.04 Bn by 2033
Recent Developments
- In August 2023, the phase II RECOVER-NEURO clinical trial study to evaluate the combination of REMOTE-transcranial direct current stimulation (tDCS) and a brain training program for long covid was launched by Soterix Medical.
- In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer’s disease. In the US, Neurocode is the first laboratory to make this test as LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Companies
- IQVIA
- Biogen
- Aurora Healthcare
- GlaxoSmithKline Plc.
- Icon Plc.
- Syneous Health
- Charles River Laboratories
- Med pace
- Covance
- Novartis AG
- Sanofi
- Merck & Co., Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Annovis Bio
- Athira Pharma, Inc.
- Zydus Group
- Eli Lilly and Company
- Eisai Co., Ltd.
- AstraZeneca
- Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
Segments Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
By Study Design
- Epilepsy
- Interventional
- Observational
- Expanded Access
- Parkinson’s Disease (PD)
- Interventional
- Observational
- Expanded Access
- Huntington’s Disease
- Interventional
- Observational
- Expanded Access
- Stroke
- Interventional
- Observational
- Expanded Access
- Traumatic Brain Injury (TBI)
- Interventional
- Observational
- Expanded Access
- Amyotrophic Lateral Sclerosis (ALS)
- Interventional
- Observational
- Expanded Access
- Muscle Regeneration
- Interventional
- Observational
- Expanded Access
- Others
- Interventional
- Observational
- Expanded Access
by Phase
- Epilepsy
- Phase I
- Phase II
- Phase III
- Phase IV
- Parkinson’s Disease (PD)
- Phase I
- Phase II
- Phase III
- Phase IV
- Huntington’s Disease
- Phase I
- Phase II
- Phase III
- Phase IV
- Stroke
- Phase I
- Phase II
- Phase III
- Phase IV
- Traumatic Brain Injury (TBI)
- Phase I
- Phase II
- Phase III
- Phase IV
- Amyotrophic Lateral Sclerosis (ALS)
- Phase I
- Phase II
- Phase III
- Phase IV
- Muscle Regeneration
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
