Real-World Data Market Key Takeaways
- North America led the market by holding 43% of market share in 2024.
- Asia Pacific is projected to grow at a solid CAGR of 11% in the coming years.
- By component, the services segment held a dominant presence in the market in 2024.
- By component, the datasets segment is expected to grow at the fastest rate during the forecast period of 2024 to 2034.
- By application, the drug development and approvals segment accounted for a considerable share of the market in 2024.
- By application, the post-market surveillance segment is anticipated to grow with the highest CAGR in the market during the studied years.
- By end-user, in 2024, the pharmaceutical and medical device companies segment led the global market.
- By end-user, the healthcare providers segment is projected to expand rapidly in the coming years.
Market Overview
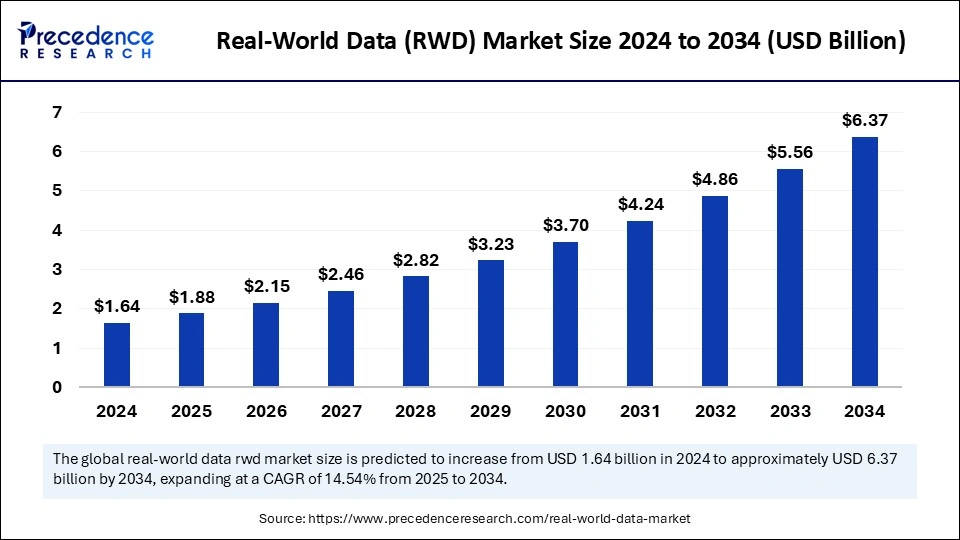
The real world data (RWD) market is expanding rapidly as healthcare providers, pharmaceutical companies, and researchers recognize the value of real world evidence (RWE) in improving patient care and accelerating drug development. Real world data encompasses data collected from various sources outside of clinical trials, such as electronic health records, insurance claims, patient registries, and mobile health devices. This data provides insights into how treatments work in real-world settings, allowing for a more comprehensive understanding of treatment outcomes and patient experiences.
The global real world data market is poised for substantial growth between 2024 and 2034, driven by increasing demand for personalized medicine, the growing adoption of digital health technologies, and the rising need for cost-effective healthcare solutions. Real world data is playing an increasingly important role in clinical research, drug development, and post-market surveillance, as it helps identify treatment patterns, assess long-term outcomes, and guide regulatory decisions. The growing emphasis on using real world evidence to support regulatory approvals is further driving the adoption of RWD solutions worldwide.
Drivers of the Real World Data (RWD) Market
-
Rising Demand for Personalized and Precision Medicine:
Personalized medicine relies on real world data to tailor treatment plans based on individual patient characteristics and responses. The ability of RWD to provide real-time insights into patient outcomes is fueling the demand for personalized healthcare solutions. -
Increased Utilization of Electronic Health Records (EHRs):
The widespread adoption of EHR systems has resulted in the generation of large volumes of patient data. This data is being harnessed to derive real world insights that enhance clinical decision-making and improve treatment outcomes. -
Regulatory Emphasis on Real World Evidence:
Regulatory authorities such as the FDA and EMA are encouraging the use of real world evidence in drug approvals and post-market surveillance. This regulatory support is driving the adoption of RWD solutions by pharmaceutical companies and healthcare organizations. -
Rising Need for Cost-Effective Healthcare Solutions:
Real world data helps healthcare providers and payers identify cost-effective treatment options by analyzing patient outcomes and healthcare utilization patterns. This focus on reducing healthcare costs is driving the demand for RWD solutions.
Opportunities in the Real World Data Market
-
Expansion of Real World Data in Oncology Research:
The application of real world data in oncology research is expanding, with researchers using RWD to evaluate treatment effectiveness, identify biomarkers, and develop targeted therapies. This trend is creating significant opportunities for market growth. -
Development of Advanced Analytics Platforms for RWD:
The development of advanced analytics platforms that integrate AI and machine learning is enhancing the ability to analyze and interpret real world data. These platforms are enabling healthcare organizations to derive actionable insights from complex datasets. -
Increasing Collaboration Between Pharmaceutical Companies and RWD Providers:
Pharmaceutical companies are increasingly partnering with real world data providers to leverage RWD for clinical research, drug development, and post-market surveillance. These collaborations are expected to drive innovation and accelerate market growth.
Challenges Facing the Real World Data Market
-
Data Privacy and Regulatory Compliance:
Ensuring compliance with data privacy regulations such as HIPAA and GDPR is a major challenge for organizations using real world data. Protecting patient confidentiality and maintaining data security are critical for the successful implementation of RWD solutions. -
Lack of Standardization in Data Collection and Analysis:
The diversity of data sources and formats in real world data presents challenges in standardizing and harmonizing datasets. Variability in data quality and completeness can impact the reliability of insights derived from RWD. -
Limited Technical Expertise and Infrastructure:
The successful implementation of real world data solutions requires specialized technical expertise and robust infrastructure. Many healthcare organizations face challenges in integrating RWD solutions into their existing systems.
Regional Analysis
North America:
North America is the largest market for real world data solutions, with the United States leading the region. The strong presence of pharmaceutical companies, advanced healthcare infrastructure, and regulatory support for real world evidence are driving market growth.
Europe:
Europe is experiencing significant growth in the real world data market, with countries such as the UK, Germany, and France investing in digital health initiatives and data-driven healthcare practices. The European Medicines Agency’s guidelines on real world evidence are encouraging the adoption of RWD solutions across the region.
Asia Pacific:
Asia Pacific is emerging as a high-growth region for the real world data market, driven by the increasing adoption of digital health technologies and rising healthcare investments. Countries such as China, India, and Japan are leveraging RWD to improve healthcare outcomes and reduce costs.
Middle East and Africa:
The Middle East and Africa region is gradually adopting real world data solutions to enhance healthcare delivery and improve patient outcomes. Government initiatives to promote healthcare digitization are supporting the growth of the RWD market in the region.
Recent News
-
In February 2025, a healthcare technology company introduced a cloud-based platform for analyzing real world data to support clinical decision-making.
-
In March 2025, a pharmaceutical giant collaborated with a real world data provider to utilize RWD in evaluating post-market drug safety.
-
In January 2025, a government healthcare agency launched a new initiative aimed at expanding the use of real world data in healthcare policy and decision-making.
Real-World Data Market Companies
- Cerner Corporation
- Evidera, Inc.
- Flatiron Health, Inc.
- IBM Corporation
- IQVIA Holdings Inc.
- Optum, Inc. (a subsidiary of UnitedHealth Group)
- Palantir Technologies Inc.
- SAS Institute Inc.
- Syneos Health Inc.
- Tempus Labs Inc.
Segments Covered in the Report
By Component
- Services
- Datasets
By Application
- Drug Development & Approvals
- Market Access & Reimbursement/Coverage Decisions
- Post-market Surveillance
- Clinical Research
- Other
By End User
- Pharmaceutical & Medical Device Companies
- Healthcare Payers
- Healthcare Providers
- Government Agencies
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!
https://www.precedenceresearch.com
