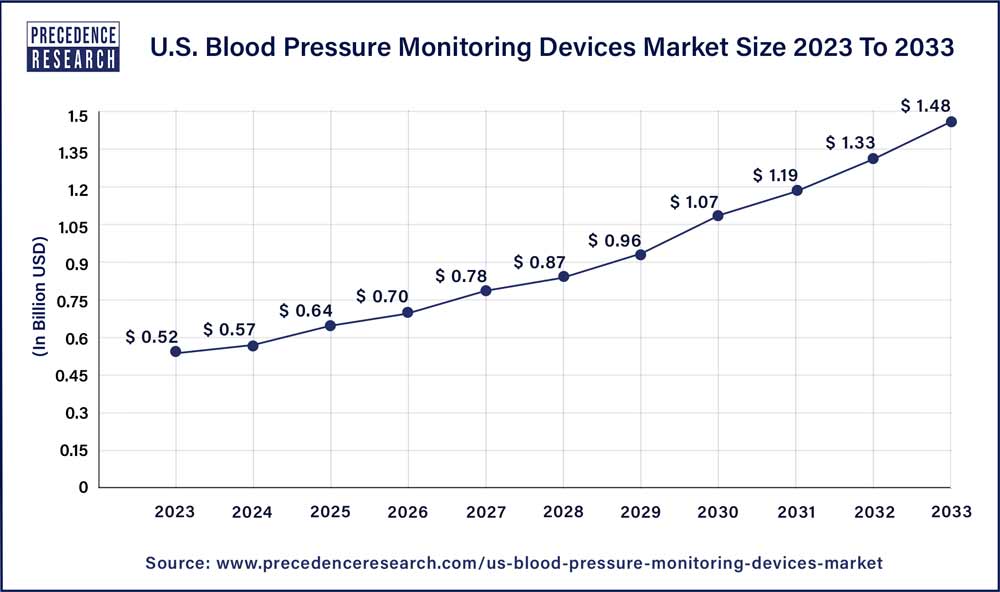
The U.S. blood pressure monitoring devices market size reached USD 0.52 billion in 2023 and is projected to hit around USD 1.48 billion by 2033, expanding at CAGR of 11.10% from 2024 to 2033.
Key Takeaways
- By product, the sphygmomanometer segment held the largest share of the market in 2023.
- By product, the ambulatory blood pressure monitor segment is expected to witness the fastest rate of expansion during the forecast period of 2024-2033.
- By end user, the hospitals segment held the largest share of the market in 2023 and the segment is observed to grow at the fastest rate during the forecast period.
- By end user, the home healthcare segment is observed to grow at the fastest CAGR during the forecast period.
Introduction
The U.S. Blood Pressure Monitoring Devices Market plays a pivotal role in the healthcare landscape, providing essential tools for monitoring and managing hypertension, a prevalent cardiovascular condition. With the increasing prevalence of lifestyle-related diseases and a growing aging population, the demand for accurate and convenient blood pressure monitoring devices has witnessed substantial growth. These devices serve as critical aids in early detection, prevention, and effective management of hypertension, contributing to improved patient outcomes and reduced healthcare costs.
Get a Sample: https://www.precedenceresearch.com/sample/3735
Growth Factors:
Several factors contribute to the continuous growth of the U.S. Blood Pressure Monitoring Devices Market. The rising incidence of hypertension, fueled by sedentary lifestyles and dietary habits, acts as a primary growth driver. Additionally, advancements in technology have led to the development of innovative, user-friendly monitoring devices, increasing patient compliance and adoption. The integration of digital health solutions, telemedicine, and home monitoring further propels market expansion, empowering individuals to actively participate in their healthcare management.
U.S. Blood Pressure Monitoring Devices Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 11.10% |
| U.S. Market Size in 2023 | USD 0.52 Billion |
| U.S. Market Size by 2033 | USD 1.48 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product and By End User |
Recent Developments
- In November 2023, the US Food and Drug Administration (FDA) authorized the Symplicity SpyralTM renal denervation (RDN) system, also referred to as the SymplicityTM blood pressure procedure, for the treatment of hypertension, according to a statement made by Medtronic plc, a pioneer in healthcare technology worldwide. Medtronic will start commercializing as soon as this permission is granted.
- In August 2023, to address health disparities and lower the risk of heart attacks and strokes in underprivileged Detroit neighbourhoods, OMRON Healthcare, a pioneer in global heart health, and EPIC Health, Detroit’s healthcare system dedicated to community-focused healthcare, launched a new partnership. Program participants will have access to VitalSightTM, OMRON’s first remote patient monitoring service created exclusively for people with uncontrolled Stage 2 hypertension and other high blood pressure patients.
U.S. Blood Pressure Monitoring Devices Market Dynamics
Drivers:
One of the key drivers accelerating the market is the increasing awareness regarding the importance of regular blood pressure monitoring for early detection and preventive healthcare. The emphasis on personalized and at-home healthcare solutions, especially in the wake of the global health challenges, has led to a surge in the adoption of blood pressure monitoring devices. The continuous efforts by healthcare providers and policymakers to promote preventive care and wellness also contribute to the market’s positive trajectory.
Restraints:
Despite the growth opportunities, the U.S. Blood Pressure Monitoring Devices Market faces certain restraints. Affordability and accessibility issues, particularly among underserved populations, pose challenges to widespread adoption. Moreover, the reliability and accuracy of some devices may vary, leading to concerns among healthcare professionals and users. Regulatory complexities and the need for standardization present additional hurdles for market players.
Opportunities:
The market is ripe with opportunities for innovation and collaboration. The integration of artificial intelligence (AI) and machine learning (ML) into blood pressure monitoring devices holds promise for enhancing accuracy and predictive capabilities. Partnerships between healthcare providers, technology companies, and insurers can create comprehensive solutions, improving patient engagement and overall health outcomes. Furthermore, expanding market reach through education and awareness campaigns can bridge gaps and unlock untapped potential.
Read Also: Hydrogen Storage Tanks and Transportation Market Size, Trend, Report 2033
U.S. Blood Pressure Monitoring Devices Market Companies
- Koninklijke Philips N.V.
- General Electric Company
- SunTech Medical, Inc.
- Welch Allyn
- American Diagnostic Corporation
- Briggs Healthcare
- Spacelabs Healthcare
- GF HEALTH PRODUCTS, INC.
- Rossmax International Limited
- Microlife Corporation
Segments Covered in the Report
By Product
- Digital Blood Pressure Monitor
- Wrist
- Arm
- Finger
- Sphygmomanometer
- Ambulatory Blood Pressure Monitor
- Instruments & Accessories
- Blood pressure cuffs
- Reusable
- Disposable
- Others
- Blood pressure cuffs
- Transducers
- Reusable
- Disposable
By End User
- Ambulatory Surgical Centers & Clinics
- Hospitals
- Home Healthcare
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
