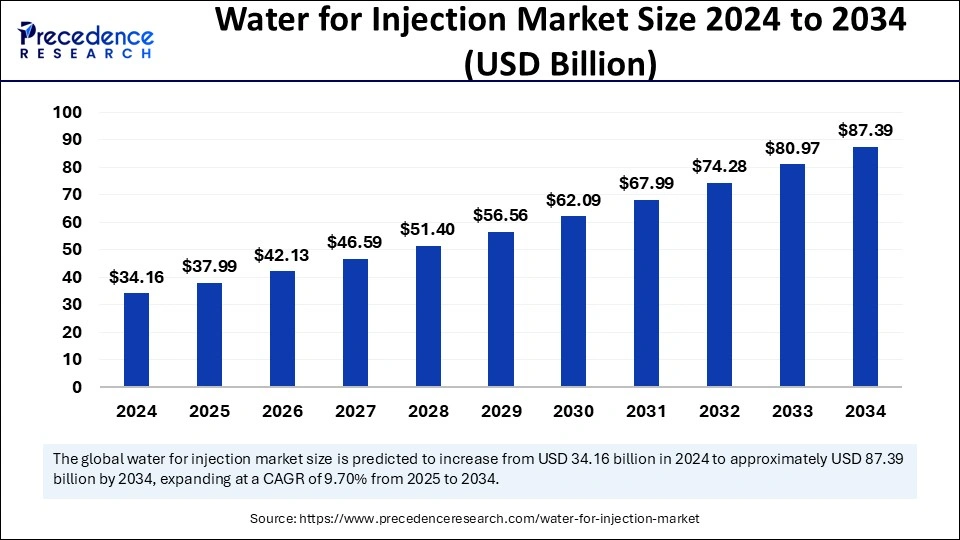
Water for injection market is estimated to grow from USD 34.16 billion in 2024 to USD 87.39 billion by 2034, at a CAGR of 9.70%.
Water for Injection Market Key Takeaways
-
In 2024, North America captured the largest share of 39.72% in the global water for injection market.
-
Asia Pacific is projected to witness strong growth, with a 10.07% CAGR over the forecast period.
-
By application, formulated parenteral drugs dominated with a 65.02% market share in 2024, while the solvent segment is anticipated to expand at a 9.76% CAGR.
-
Pharmaceutical and biotechnology companies accounted for the biggest market share of 43.05% by end-user in 2024.
-
The research institutes segment is expected to register the highest growth rate of 9.73% during the forecast period.
Water for Injection Market Overview
The Water for Injection (WFI) Market is expanding rapidly due to advancements in water purification technologies and the increasing demand for ultra-pure water in pharmaceutical and biopharmaceutical manufacturing. WFI is an essential component in the production of parenteral drugs, biologics, and cell-based therapies, requiring compliance with strict regulatory guidelines.
Recent technological innovations such as vapor compression distillation, reverse osmosis, ultrafiltration, and multiple-effect distillation (MED) have enhanced the efficiency, reliability, and scalability of WFI production systems. As the pharmaceutical industry embraces continuous manufacturing and single-use technologies, the demand for high-quality WFI is expected to grow further, driving market expansion.
Water for Injection Market Drivers
-
Advancements in Water Purification Technologies
Technological innovations in distillation, ultrafiltration, and membrane-based purification processes are enhancing the efficiency and cost-effectiveness of WFI production, reducing operational complexity and ensuring compliance with regulatory standards. -
Growing Demand for Single-Use and Continuous Manufacturing Systems
The adoption of single-use bioreactors and continuous manufacturing systems in biopharmaceutical production is increasing the demand for reliable and consistent WFI supply. These technologies require ultra-pure water to maintain product quality and integrity. -
Increased Regulatory Emphasis on Process Validation and Quality Control
Regulatory agencies are emphasizing stringent validation, monitoring, and quality control protocols for WFI production. Pharmaceutical companies are investing in advanced water purification systems to meet these evolving regulatory requirements.
Water for Injection Market Opportunities
-
Emergence of Alternative WFI Production Methods
The development of alternative WFI production methods, such as reverse osmosis and ultrafiltration, offers cost-effective and sustainable solutions for pharmaceutical manufacturers. These methods reduce energy consumption and operational costs, presenting growth opportunities for market players. -
Integration of IoT and Automation in WFI Systems
The integration of IoT-enabled sensors, real-time monitoring, and automated control systems in WFI production processes enhances process efficiency, reduces downtime, and ensures consistent water quality. -
Adoption of Green Technologies and Sustainable Practices
Pharmaceutical companies are increasingly focusing on reducing their environmental footprint by adopting green technologies and sustainable practices in WFI production. The implementation of energy-efficient distillation systems and membrane-based purification technologies is driving this trend.
Water for Injection Market Challenges
-
Complexity in Scaling Up WFI Production
Scaling up WFI production to meet the growing demand requires sophisticated process control, validation, and quality assurance systems. Ensuring consistency and compliance at higher production scales poses a significant challenge. -
High Energy and Operational Costs in Distillation-Based Systems
Traditional distillation-based WFI production systems consume large amounts of energy, increasing operational costs. Pharmaceutical companies are seeking alternative methods that reduce energy consumption without compromising water quality. -
Challenges in Implementing Continuous Monitoring and Validation
Ensuring continuous monitoring and real-time validation of WFI systems to prevent microbial contamination and endotoxin presence requires robust analytical tools and process control mechanisms.
Water for Injection Market Regional Insights
-
North America
North America is a leader in adopting advanced water purification technologies, driven by the presence of leading pharmaceutical and biotechnology companies and stringent regulatory requirements. -
Europe
Europe is a significant market for WFI, with regulatory agencies emphasizing the adoption of high-quality water systems in pharmaceutical production. The region is witnessing increased investments in advanced water purification systems. -
Asia Pacific
The Asia Pacific region is emerging as a lucrative market due to rapid growth in pharmaceutical manufacturing, increasing demand for biologics, and expanding investments in healthcare infrastructure.
Water for Injection Market Recent Developments
-
Introduction of IoT-Enabled WFI Monitoring Systems
Market players are launching IoT-enabled WFI monitoring systems that provide real-time data on water quality, ensuring compliance and operational efficiency. -
Adoption of Energy-Efficient Distillation Technologies
Manufacturers are introducing energy-efficient distillation technologies that reduce operational costs and environmental impact, enhancing sustainability in WFI production. -
Strategic Alliances to Expand WFI Production Capacity
Key players are entering strategic alliances and partnerships to increase their WFI production capacity and cater to the growing demand from pharmaceutical manufacturers.
Water for Injection Market Companies
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Eurocrit Labs International
- Evoqua Water Technologies
- Veolia Water Solutions and Technologies
- ICU Medical Inc.
- Danaher Corporation (Cytiva)
- Rocky Mountain Biologicals
- B. Braun Melsungen AG
- SteriCare Solutions
- Veltek Associates, Inc.
Segment Covered in the Report
By Application
- Formulate Parental Drugs
- Solvent
- Cell Culture Media
- Laboratory Reagents
- Synthesis of Drugs
- Others
- Cleaning Agents
- Rinsing Vessels
- Cleaning Equipment
- Cleaned primary Packaging Materials
By End-user
- Pharmaceutical and Biotechnology Companies
- Research Institutes
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!
